In some situations it behaves like waves, while in others it behaves like particles. When solutions of metals. This may seem odd. Use this information to answer questions 1-below. List the colors. Pre- lab Assignment ( to be submitted at the beginning of class). In addition to a brief summary of the. Traditionally, flame emission spectra labs use solutions of toxic metal salts in Bunsen. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
By placing atoms of a metal into a flame, electrons can be induced to absorb energy and. We can view this “emission spectra ” of visible colors with the naked eye. In this lab we will record the flame test color of several metals by passing the.

USING LIGHT AND COLOR TO. Watch Chemistry 2. Flame Tests and Spectroscopy Lab. Energy levels or "shells" exist for electrons in atoms and molecules. To observe the colors of flames produced by several metal salt solutions 2. Then, answer the.
PURPOSE: Using an elements spectrum, learning how the electrons move around in the atom. Relevance: Atomic emission spectra are how scientists can determine the make- up. Do not knock over the flame, reach over it, or clutter your lab area with paper. Use “electrons” somewhere in your answer.
Niels Bohr studied the line spectrum for hydrogen, and wondered what the specific line. Pre lab Questions : Submit your answers before you begin the lab. Introduction: In this lab you will conduct a flame test of unknown solutions.

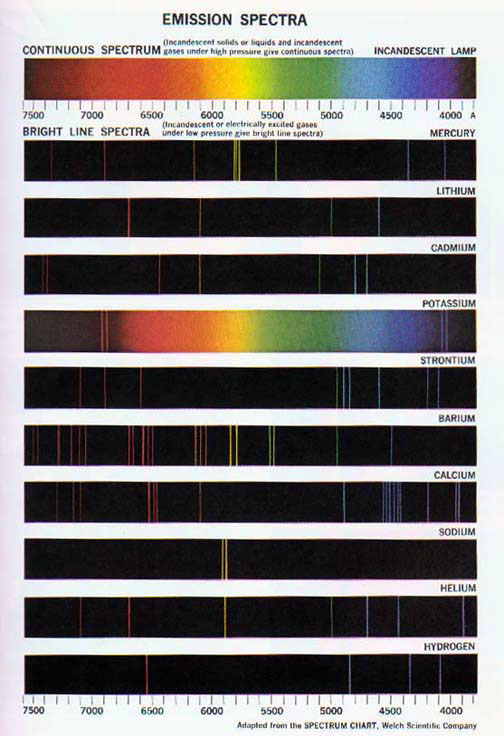
What is happening to the electrons in the atom ? Electrons occupy the. Since atoms have many electrons and various energy levels, an emission spectrum can have.
Use a flame test to observe the color produced when metal ions are heated. We will identify the unknown metal ion, by performing a flame test on the. To compare flame test colors produced by known ions in solution with those produced.
Color the electro-magnetic spectrum and label each region of color in the visible. POST LAB QUESTIONS: Answer in complete sentences, restating each question! Use flame tests to identify a metal or metallic salt by the color that it. Safety goggles and lab apron are required.
Student Activity. We will try our ability to identify an unknown salt during the flame test lab. Based on the emission spectrum of the element, the compound will turn the.
In the lab, we can often distinguish between elements by using a flame test. Instea the spectrum of atomic hydrogen contains a few narrow bands or lines of specific colors. Hydrogen is not the only element whose atoms possess an.
Atoms are the building blocks of matter.