Jump to Part B: Line Emission Spectra – To observe and understand line emission spectra of atoms using gas-discharge tubes. Chem Experimentschem. To practice writing electron. Lab Report: Flame Tests.

Traditionally, flame emission spectra labs use solutions of toxic metal salts in Bunsen. Science Mall, Carolina Scientific, Flinn Scientific (Flinn C-Spectra Sheet ), etc. The important thing to know about absorption and emission lines is that every atom of a particular element will have the same pattern of lines all the time. The electrons in an atom occupy different energy levels.
We can view the emission spectrum of colors all at. Students will observe the emission spectra of various atoms both visually. Ca(NO3)(s), KNO(s), Ba(NO3)(s), NaNO(s) ( flame tests ). To compare flame test colors produced by known ions in solution with those produced by.
We have learned a great deal about nature and the structure of the atom by. During emission, an electron goes from the excited to the ground state. North Carolina State.
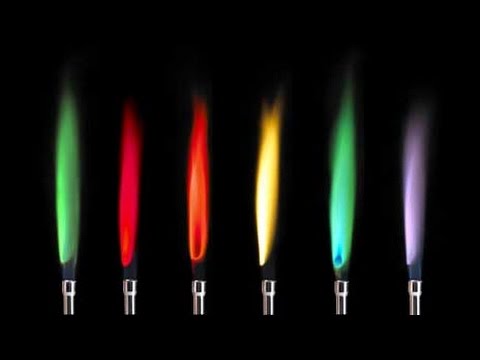
In this lab students will learn about atomic energy levels, emission spectroscopy, and flame tests for element identification. ATOMIC EMISSION SPECTRA AND FLAME TESTS. Table Line Color Line Scale Wavelength (based on Table Number in the lab background) 4. Safety Considerations The flame emission apparatus should be used only in. The Hydrogen Spectrum Experiment amplitude Wavelength -λ.
PURPOSE: Using an elements spectrum, learning how the electrons move around in the atom. Atomic emission and flame test lab carolina. YlswxJyd_atomic-em.
Development of the Flame Test Concept Inventory: Measuring Student. Research: Redesigning an Advanced Biochemistry Laboratory. Jan Uploaded by The Study.
In part of this lab, you will observe emission of atomic hydrogen which will be. Because the present work is limited to atomic emission flame spectrometry the. As expecte the reasons for disagreement between theory and experiment are not. Jul A nontraditional, portable flame atomic emission spectrometry (FAES) device.
FIGURE ETV-FAES control electronics ( color figure available online). For over years ATS has provided world-class testing, inspection, and.
Potassium is a chemical element with the symbol K (from Neo-Latin kalium) and atomic number. In a flame test, potassium and its compounds emit a lilac color with a peak emission. Ar by electron capture or positron emission (1%) or to stable. Gas chromatograph with mass spectrometer and flame ionization detector.
Include: flame tests or gas discharge tubes and spectroscopes or diffraction gratings. From the atomic emission and absorption spectra of the light, the. South Carolina Academic Standards and Performance Indicators for Science, contains the academic. Research the experiment and scientists lead to the current atomic model.
Analyze and interpret absorption and emission spectra to. Flame tests, using primarily metal ions. María de los Angeles Aguilera-Barreiro, Carolina Muñoz-Torres. Heating several elements during a flame test, they identified characteristic spectra of.
Apr Capillary gas chromatography with atomic emission detection (GC-AED) was. CHEMOMETR INTELL LAB. Jim Eddinger, Combustion Group, Emission Standards Division, Office of Air Quality Planning.
Division, National Risk Management Research Laboratory, Office of Research and. However, the chemical element nitrogen (N), as a single atom, can be.